Lithium iron phosphate is one of the most important materials for batteries in electric cars, stationary energy storage systems and tools. It has a long service life, is comparatively inexpensive and does not tend to spontaneously combust. Energy density is also making progress. However, experts are still puzzled as to why lithium iron phosphate batteries undercut their theoretical electricity storage capacity by up to 25% in practice.
In order to utilize this dormant capacity reserve, it would be crucial to know exactly where and how lithium ions are stored in and released from the battery material during the charging and discharging cycles.
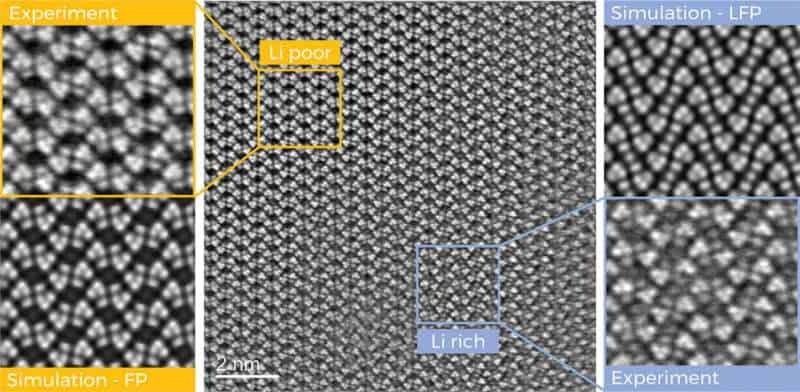
Researchers at Graz University of Technology (TU Graz) have now taken a significant step in this direction. Using transmission electron microscopes, they were able to systematically track the lithium ions as they traveled through the battery material, map their arrangement in the crystal lattice of an iron phosphate cathode with unprecedented resolution and precisely quantify their distribution in the crystal.
Their study is published in the journal Advanced Energy Materials.
Key clue for increasing the capacity of batteries further
“Our investigations have shown that even when the test battery cells are fully charged, lithium ions remain in the crystal lattice of the cathode instead of migrating to the anode. These immobile ions incur a cost in capacity,” says Daniel Knez from the Institute of Electron Microscopy and Nanoanalysis at TU Graz.
The immobile lithium ions are unevenly distributed in the cathode. The researchers have succeeded in precisely determining these areas of different levels of lithium enrichment and separating them from each other down to a few nanometers.
Distortions and deformations were found in the crystal lattice of the cathode in the transition areas.
“These details provide important information on physical effects that have so far counteracted battery efficiency and which we can take into account in the further development of the materials,” says Ilie Hanzu from the Institute of Chemistry and Technology of Materials, who was closely involved in the study.
Methods are also transferrable to other battery materials
For their investigations, the researchers prepared material samples from the electrodes of charged and discharged batteries and analyzed them under the atomic-resolution ASTEM microscope at TU Graz. They combined electron energy loss spectroscopy with electron diffraction measurements and atomic-level imaging.
“By combining different examination methods, we were able to determine where the lithium is positioned in the crystal channels and how it gets there,” explains Nikola Šimić from the Institute of Electron Microscopy and Nanoanalysis, the first author of the paper.
“The methods we have developed and the knowledge we have gained about ion diffusion can be transferred to other battery materials with only minor adjustments in order to characterize them even more precisely and develop them further.”


























