Professor Soojin Park, Seoha Nam, a Ph.D. candidate, and Dr. Hye Bin Son from the Department of Chemistry at Pohang University of Science and Technology (POSTECH) have achieved a breakthrough in creating a gel electrolyte-based battery that is both stable and commercially viable. Their research was published in the journal Small.
Lithium-ion batteries are extensively utilized in portable electronics and energy storage, including electric vehicles. However, the liquid electrolytes used in these batteries pose a significant risk of fire and explosion, prompting ongoing research efforts to find safer alternatives.
One alternative is the semi-solid-state battery, which represents a middle ground between traditional lithium-ion batteries with liquid electrolytes and solid-state batteries. By using a gel-like electrolyte, these batteries offer enhanced stability, energy density, and a relatively longer lifespan.
Creating gel electrolytes typically involves a prolonged heat treatment at high temperatures, which can degrade the electrolyte, leading to diminished battery performance and increased production costs. Additionally, the interface resistance between the semi-solid electrolyte and the electrode poses a challenge in the fabrication process.
Previous studies have encountered limitations in applying their findings directly to current commercial battery production lines due to complex fabrication methods and issues with large-scale applications.
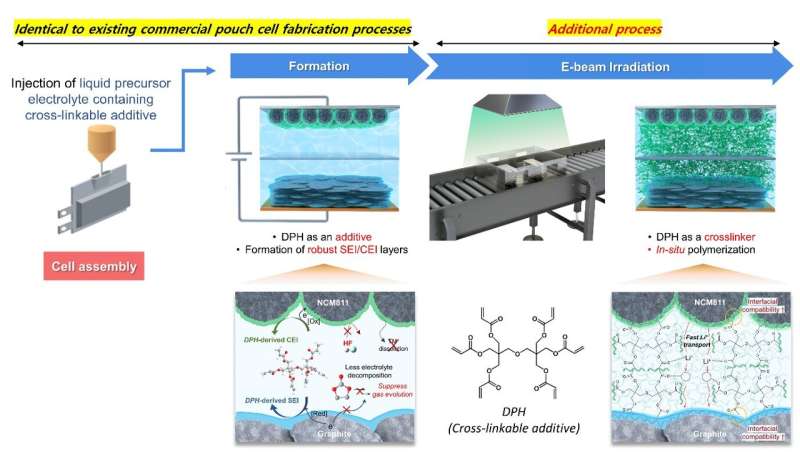
Professor Soojin Park’s team tackled these challenges using a bifunctional cross-linkable additive (CIA), dipentaerythritol hexaacrylate (DPH), combined with electron beam (e-beam) technology.
The conventional pouch-type battery manufacturing process includes the electrode preparation, electrolyte injection and assembly, activation, and degassing steps. However, the researchers enhanced DPH’s dual functionality by simply introducing an additional e-beam irradiation step after the degassing process.
The CIA acted as both an additive to facilitate a stable interface between the anode and cathode surfaces during activation and as a crosslinker to form a polymer structure during the e-beam irradiation process.
The team’s pouch-type battery, employing a gel electrolyte, significantly reduced gas generation from battery side reactions during initial charging and discharging processes, achieving a 2.5-fold decrease compared to conventional batteries. Furthermore, it effectively minimized interfacial resistance due to strong compatibility between electrodes and the gel electrolyte.
Subsequently, the researchers developed a high-capacity battery of 1.2 Ah (ampere-hour) and tested its performance at 55 degrees Celsius, an environment that accelerates electrolyte decomposition. In this condition, batteries using conventional electrolytes experienced substantial gas generation, leading a rapid reduction in capacity with swelling of the battery after 50 cycles.
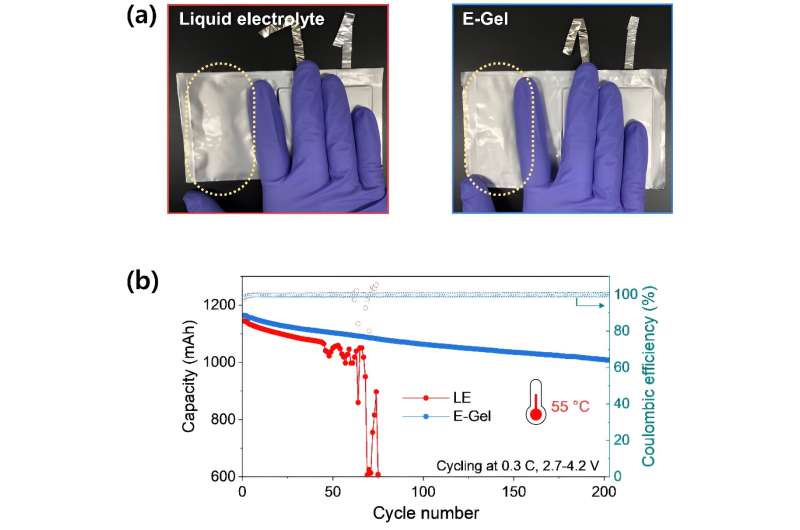
In contrast, the team’s battery showed no gas generation and maintained a 1 Ah capacity even after 200 cycles, demonstrating its enhanced safety and durability.
This research is particularly significant because it enables both the safety and commercial viability of gel electrolyte-based batteries to be rapidly mass-produced within existing pouch battery production lines.
Professor Soojin Park of POSTECH said, “This achievement in stability and commercial viability is poised to be a breakthrough in the electric vehicle industry. We hope this advancement will greatly benefit not only electric vehicles but also a wide range of other applications that rely on lithium-ion batteries.”


























