Scientists at EPFL have developed advanced atom-thin graphene membranes with pyridinic-nitrogen at pore edges, showing unprecedented performance in CO2 capture. It marks a significant stride toward more efficient carbon capture technologies.
As the world battles climate change, the need for efficient and cost-effective carbon capture technologies is more urgent than ever. In that vein, scientists are exploring a number of innovations to drastically reduce industrial carbon emissions, which is pivotal in mitigating global warming.
One of these is carbon capture, utilization, and storage (CCUS), a critical technology that reduces carbon dioxide (CO2) emissions from hard-to-abate industrial sources such as power plants, cement factories, steel mills, and waste incinerators. But current capture methods rely on energy-intensive processes, which makes them costly and unsustainable.
Research now aims to develop membranes that can selectively capture CO2 with high efficiency, thereby reducing the energy and financial costs associated with CCS. But even state-of-the-art membranes, such as polymer thin films, are limited in terms of CO2 permeance and selectivity, which limits their scalability.
So the challenge is to create membranes that can simultaneously offer high CO2 permeance and selectivity, crucial for effective carbon capture.
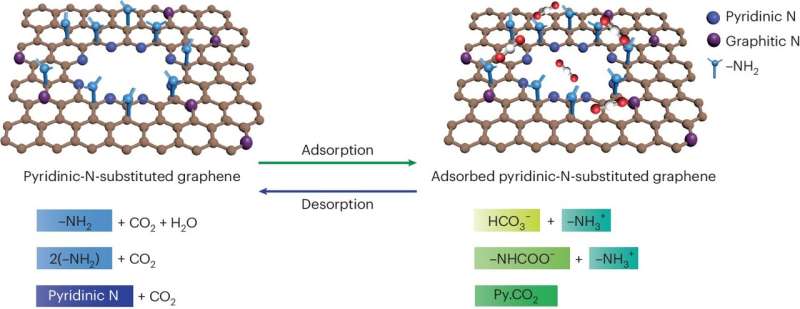
A team of scientists led by Kumar Varoon Agrawal at EPFL has now made a breakthrough in this area by developing membranes that show exceptional CO2 capture performance by incorporating pyridinic nitrogen at the edges of graphene pores.
The membranes strike a remarkable balance of high CO2 permeance and selectivity, making them highly promising for various industrial applications. The work is published in Nature Energy.
The researchers began by synthesizing single-layer graphene films using chemical vapor deposition on copper foil. They introduced pores into the graphene through controlled oxidation with ozone, which formed oxygen-atom functionalized pores. They then developed a method to incorporate nitrogen atoms at the pore edge in the form of pyridinic N by reacting the oxidized graphene with ammonia at room temperature.
The researchers confirmed the successful incorporation of pyridinic nitrogen and the formation of CO2 complexes at the pore edges by using various techniques, such as X-ray photoelectron spectroscopy and scanning tunneling microscopy. The incorporation of pyridinic N remarkably improved the binding of CO2 on graphene pores.
The resulting membranes showed a high CO2/N2 separation factor, with an average of 53 for a gas stream containing 20% CO2. Remarkably, streams with about 1% CO2, achieved separation factors above 1,000 because of the competitive and reversible binding of CO2 at the pore edges, facilitated by the pyridinic nitrogen.


























