Reliably monitoring the amount of lithium (Li) present in rechargeable batteries, specifically in the so-called cathode active material (CAM), is key to understanding the condition of batteries from the time when it is fabricated to the end of its operation. However, a reliable methodology to directly track the active Li in batteries without damaging them does not exist yet.
Existing methods to estimate the amount of Li in batteries rely on capacity measurements, describing how much charge a battery can hold, and coulombic efficiency values, which indicate how much charge a battery retains during cycles. Yet these measurements are not always accurate, as they do not account for unpredictable side reactions, self-discharge, and other effects affecting a battery’s performance.
Researchers at Idaho National Laboratory and Binghamton University recently set out to test the performance of Li-battery cells with different formulations and underlying configurations under different conditions. Based on their findings, published in Nature Energy, the team devised a new framework that could help to reliably compare data collected when testing rechargeable batteries to results obtained during their real-world operation in different conditions.
“The primary objective of our study was to find a reliable methodology to compare battery testing data and operating results from various sources and conditions, as this could help to advance battery technology and development,” Boryann Liaw, co-author of the paper, told Tech Xplore.
“The conventional battery capacity analysis is empirical, heavily relying on test protocols and conditions, lacking a reliable framework for comparison. This work provides a thermodynamic framework and methodology that can compare data from across the board consistently.”
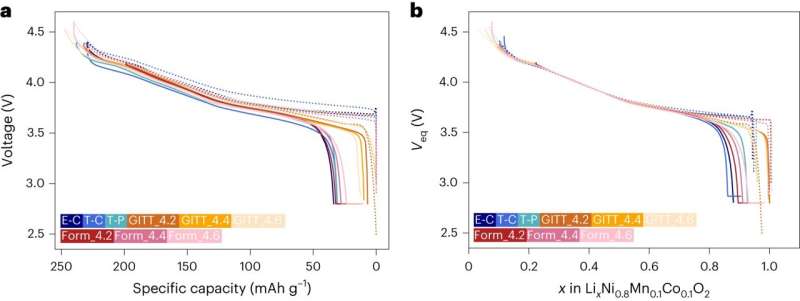
Leveraging the theoretical capacity of a transition metal oxide, the researchers were able to reliable estimate how much Li was in a battery’s electrode. This allowed them to monitor small changes in the composition at the interface between a battery’s electrode and electrolyte.
As part of their study, Liaw and his colleagues used a total of 12 Li-NMC (layered transition metal oxide cathode material) cells with various chemical formulations and structural configurations, under a wide range of conditions. The results they collected were used to demonstrate a matrix that is traditionally difficult to perform, outlining a quantitative comparison among these cells.
“We introduced a reliable fundamental thermodynamic framework and methodology that can compare various battery test data from across the board in a rationalized quantitative manner,” Liaw said. “This approach can substantially reduce resources for battery testing and time to market, enable effective and reliable battery manufacturing, operating, and management strategies, and provide a secured and safe supply chain.”
The recent framework devised by Liaw and his collaborators sheds further light on variables affecting the performance of battery cells and their electrodes over time. In the future, it could help other research groups to optimize their battery technologies, improving their performance and reliability. This could in turn prompt to the introduction of new promising rechargeable Li batteries with higher storage capacities and longer lifespans.
“We now plan to work with academia and industry partners to broaden the applications of this approach to help battery research and development efforts,” Liaw added.





























