The sustainably powered, electrochemical reduction of carbon dioxide (CO2) into useful chemicals and feedstock could help to mitigate greenhouse gas emissions, allowing industries to reuse released CO2 in beneficial ways. Most of the strategies for realizing this introduced so far, however, have notable limitations, including a poor stability over long periods of time.
Researchers at Hong Kong Polytechnic University, University of Oxford and the National Synchrotron Radiation Research Center recently introduced a new membrane-electrode-assembly system that could facilitate the stable electrocatalytic reduction of CO2.
Notably, their proposed system, which was first introduced in a paper published in Nature Energy, is fed by pure water (H2O) and thus does not rely on alkali-metal electrolyte.
“Our modern society heavily depends on fossil fuels to power our economy, but the resulting CO2 emissions are a major threat to the climate,” Shu Ping Lau, co-author of the paper, told Tech Xplore. “We are looking to harness and re-enter massive amounts of CO2 into the carbon cycle by using electrocatalytic CO2 reduction (ECO2R) technology to combat this. However, previous research has shown that the stability of the ECO2R system is a major challenge, with current systems lasting less than 200 hours for ECO2R-to-ethylene (C2H4).”
In their recent work, Lau and his collaborators have been trying to overcome the limitations of existing systems for electrocatalytic CO2 reduction. Their objective is to create a new electrolysis architecture that suppresses carbonate formation during ECO2R and thus enables a prolonged stable operation.
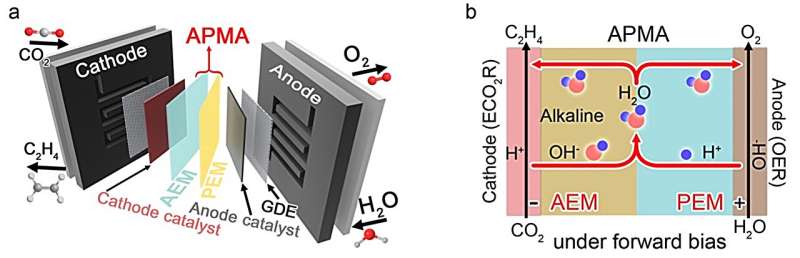
“Our goal is to maintain an alkaline cathode environment without involving alkali metal cations, ultimately designing the APMA MEA (AEM+PEM assembly membrane-electrode assembly) architecture with pure H2O as the anolyte,” Lau explained. “In our APMA MEA ECO2R system, we’ve created a way for CO2 to react with H2O to produce C2H4 and OH– at the cathode, while H2O is oxidized into O2 and H+ at the anode. The resulting OH– and H+ then combine to form H2O in the middle of the membranes.”
The new system introduced by the researchers is comprised of two distinct membranes (AEM and PEM), a cathode catalyst (stepped-surface Cu), an anode catalyst (Pt/Ti) and pure water as the anolyte. One of its most remarkable advantages is that it does not require any additional chemicals to initiate reactions, and just uses pure H2O as the electrolyte. This means that it could be easily scaled up to an industrial level.
“Even more impressive, the APMA MEA architecture overcomes the thermodynamic limitation of CO2 reacting with the electrogenerated OH- into carbonate, which extends the system’s stability,” Lau said. “With its durability and efficiency, our APMA MEA system has the potential to revolutionize CO2 electrocatalysis technology and transform the modern fossil energy system.”




























