Energy storage is an essential element of the clean energy transition, from the electrification of cars to helping smooth out the intermittency of variable renewables such as wind and solar. Lithium-ion (Li-ion) batteries are already one of the most prominent energy storage options due to their high energy density and relatively low and declining cost. There also seems to be no end to the growth in types of electronic devices, from mobile phones to laptops, making similar demands on these battery types.
However, despite electrochemistry specialists and battery manufacturers delivering steady improvements over the years, even state-of-the-art Li-ion batteries continue to struggle to support many heavy-duty energy storage applications. This is in part due to their short calendar life.
A battery whose capacity (the total amount of electricity it is able to produce) has decreased to 80% of its initial capacity is said to have reached the end of its calendar life. For heavy-duty applications such as grid-scale energy storage, in order to avoid the high costs of replacement, the calendar life of Li-ion batteries needs to be around 15–20 years following installation.
But the technology is still far from delivering on that promise. Researchers say that for heavy-duty energy storage to be more of a commercial success, investigation into the causes of lithium battery corrosion and how to inhibit this need much closer attention.
They published their findings in Nano Research Energy on December 09, 2022.
Like all batteries, the calendar life of lithium batteries, including Li-ion batteries, is dictated by how stable (resistant to degradation) they are during storage and cycles of charging.
And cycling stability in turn depends on the stability of the anode (the negative electrode), cathode (the positive electrode), and electrolyte (the medium that provides the transport mechanism for ions between the electrodes)—both at the interfaces between these battery components and in their bulk material (main part of their mass).
An enormous amount of effort by electrochemists has gone into optimizing bulk material structure, modifying interfaces, and designing better electrolytes in order to improve cycling stability.
“But comparatively less effort has gone into improving the second element that determines calendar life: storage stability,” said Xue-Qiang Zhang the paper author from Beijing Institute of Technology, “and how this is undermined by corrosion.”
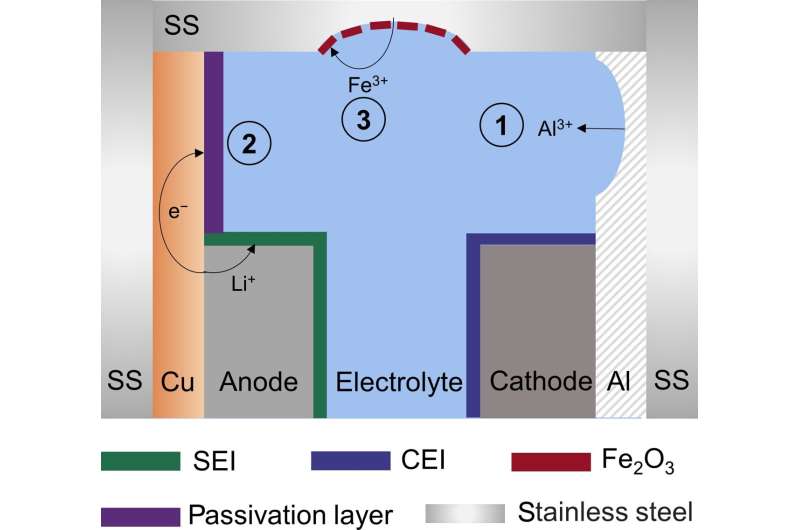
Lithium batteries can spend a long-time storing energy and not cycling through. During storage, there are various unwanted chemical reactions that result in the deterioration of components for many reasons, not least the high reactivity of electrode materials, and the incompatibility between the element that collects the electric current and electrolytes. This deterioration—also known as corrosion—degrades the structural stability of the batteries, ultimately shortening calendar life.
As a result, any effort at improving storage stability must focus on a better understanding of the mechanisms of corrosion and developing strategies to inhibit it.
“We wanted to give an overview of the current state of understanding of corrosion and storage stability so as to better understand and tackle research gaps,” added Jia-Qi Huang also a paper author from Beijing Institute of Technology, “Corrosion remains a largely unsolved issue in lithium batteries of all types.”
Having surveyed the scientific literature on the topic, the authors concluded that corrosion reactions in Li batteries primarily involve three aspects: the electrochemical corrosion of aluminum current collector, electrochemical corrosion of the stainless-steel case that encloses the battery, and the galvanic corrosion (when one metal corrodes more than another metal with which it is in electrical contact, and both are immersed in the electrolyte solution) of the anode. Ultimately, the corrosion originates with the chemical and electrochemical reactions between electrode materials and the electrolytes.
Researchers have so far focussed on three main strategies for inhibiting corrosion: attempting to better regulate electrolyte decomposition reactions; isolating the electrode materials from the electrolytes by some forms of artificial coating; and trying to lower the reactivity of electrode materials via modifying their surfaces.
The authors offered five main recommendations to give a boost to research into lithium battery storage corrosion issues.
First, much more work needs to be performed investigating galvanic corrosion, which is common in lithium batteries. There are few effective strategies to mitigate this at present. Surface modification of the copper current collector is one possible strategy worth investigating. This might be achieved by the use of electrolyte additives. Development of a protective surface coating for the copper foil might be another approach.
Second, any future improvement strategies need to be evaluated under realistic conditions of temperature, humidity, and so on, not just in the lab. The authors found that most novel corrosion inhibition strategies have generally been evaluated under very mild environmental conditions in the laboratory, not in the real world.
Relatedly, a third strategy would focus on accelerating these evaluation methods. Corrosion is normally a slow process, so evaluation is necessarily time-consuming and thus costly. Figuring out a way to speed this up is essential.
Alongside real-world observation, researchers should embrace real-time monitoring methods to capture an understanding of corrosion in working batteries. This should enable a greater ability to recognize the healthy state of the battery, and thus more ably predict battery life and avoid sudden battery failure.
Finally, and exacerbating all these problems, new battery designs are continuously emerging. New electrode materials and electrolytes are constantly being developed. Cycling performance of these novel designs is regularly tested, but not their impact on corrosion. Yet such new materials potentially alter the corresponding corrosion mechanisms and thus require alterations to corrosion inhibition strategies.
The authors of the review hope that once battery researchers embrace their recommendations, some real breakthroughs countering lithium battery corrosion and thus extending calendar life can be made.





























