For many of the world’s most widespread and infectious diseases, several effective treatments are already available, approved and known of, but are in need of improvements upon delivery, dosage precision, and supply chain management. One company has emerged as leading candidate to improve upon these treatments through drug repurposing and patented technologies proven to make available medicines much, much better.

Mountain Valley MD (CSE:MVMD) (OTC:MVMDF) is a biotech company with multiple patented streamlined approaches to addressing some of the world’s biggest medical challenges by repurposing and reformulating existing medical and non-medical compounds (drugs, vaccines, nutraceuticals, etc.). The company also has a potentially game-changing, enhanced end-to-end supply chain encompassing the formulation, stabilization, storage, solubility and unique delivery methods of compounds.
60-Second Streamlined Summary
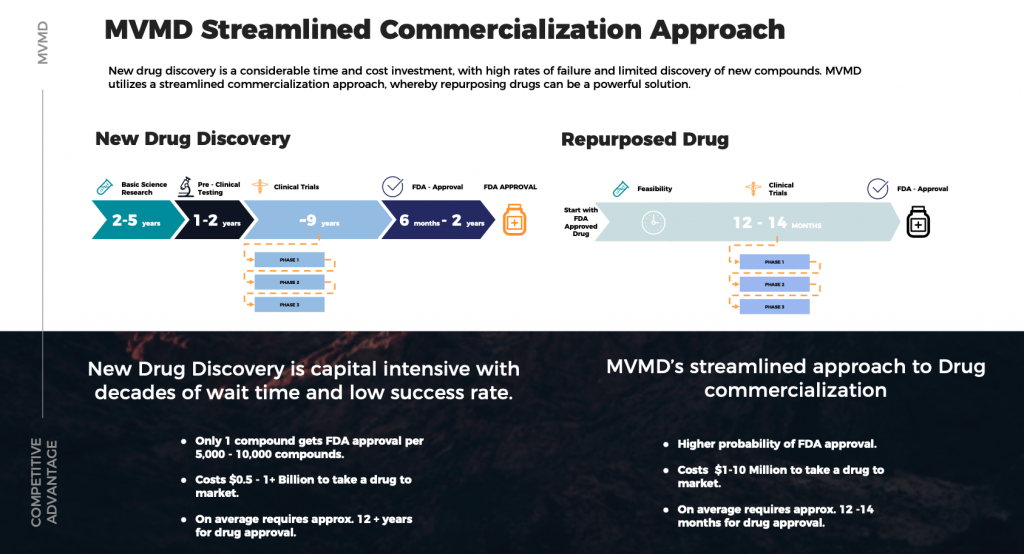
- Drug repurposing offers advantages over developing an entirely new drug by identifying new uses for approved or investigational drugs outside of their original scopes of medical indication.
- Mountain Valley MD (CSE:MVMD) (OTC:MVMDF) has developed a streamlined, cost-effective approach to gaining commercialization approvals.
- THREE Patented Technologies that improve absorption, dose delivery, and end-to-end delivery that reduces and/or eliminates costly cold chain logistics
- Reduced dosages, Faster Absorption, and Precision Dosing, thus eliminating variability, simplifying administration, minimizing side effects, and increasing bioavailability
- Preclinical Trial Data delivered an 800% increase in bioavailability through intramuscular (IM) injection, and 500% increase in bioavailability through sublingual compared to oral tablets.
- Global Drug Delivery Systems (DDS) Markets forecasted to hit $158 Billion by 2027[1]
- Addressing Cold Chain Supply Issues, such as $35 billion dollars a year in annual disposal of drugs/vaccines that break cold chain and/or don’t have the confidence to deliver[2], and global spending on pharma cold chain is expected to reach $21.3 billion by 2024 at a CAGR of 24% along the way[3]
- High Potential and Industry Anticipation for utilizing drug repurposing to combat both Cancer[4],[5] and COVID-19[6],[7]
- Premium Management Team with a strong pedigree and track record of successes in product development and launches
Prior to the Sars-COV-2 (COVID-19) outbreak, many people remembered the legendary success story of Pfizer and one of the most famous success stories of all time involving drug repurposing—the story of the discovery of Viagra.
Originally developed as an angina medication for sufferers of heart challenges, upon the discovery of a very unique side effect, the little blue pill was repurposed.
A new application was quickly submitted, and within 4 years of this discovery Sildenafil would go on to become Viagra the best-selling erectile dysfunction medication of all time[8] and making Pfizer tens of billions of dollars along the way between its 1998 approval and 2020 patent expiration date.[9]
Even after many new competitors and generics rushed to fill the market, Viagra still generates close to half a billion annually for the drug giant.
Drug repurposing (aka drug repositioning, reprofiling or re-tasking) is a common practice, that when done correctly can be not only quite lucrative, but also quite beneficial to recipients of the newly formulated versions of the medicine.
This is where Mountain Valley MD (CSE:MVMD) (OTC:MVMDF) comes with a distinct advantage among its pharma peers. With multiple patents in hand and even more pending, the biotech developer has the tools in place to repurpose already-approved drugs, in order to make them more effective, more precise, and safer.
And they can do this with a strategy that’s not only more cost-effective than bringing a new drug to market, but also in a fraction of the time.
MVMD has already grown to a market cap of +$100 million and built up a portfolio of 16 Patents/Patents Pending across various delivery technologies, for repurposing of compounds.
Now, Mountain Valley MD (CSE:MVMD) (OTC:MVMDF) is poised for a massive breakthrough, by targeting the repurposing of proven drugs to tackle some of the world’s most concerning illnesses, including cancer and COVID-19.
By implementing enhancements to the solubility of these drugs, as well as introducing a new adjuvant technology that could save the pharmaceutical industry BILLIONS in waste, by reducing reliance on cold chain logistics and dose spoilage.
In short: MVMD makes existing drugs and drug supply chains BETTER.
“The extrapolation of this technology achievement across multiple viral applications could be very significant and has the potential to positively impact human and animal health globally.”
– Dennis Hancock, President & CEO of Mountain Valley MD

MVMD’s Approval Advantage
MVMD’s strategic pathway leverages the Federal Food, Drug and Cosmetic Act provision 505b(2): repurposing active ingredients presently in existing drug products for novel uses.
The provision allows MVMD to bridge the gap between what is already known in the biotechnology space to further innovate.
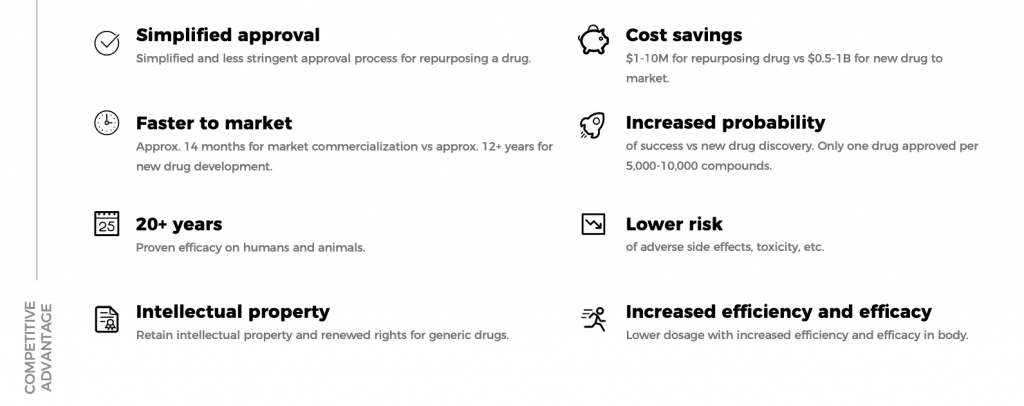
At the forefront of the MVMD advantage are its three flagship technologies:
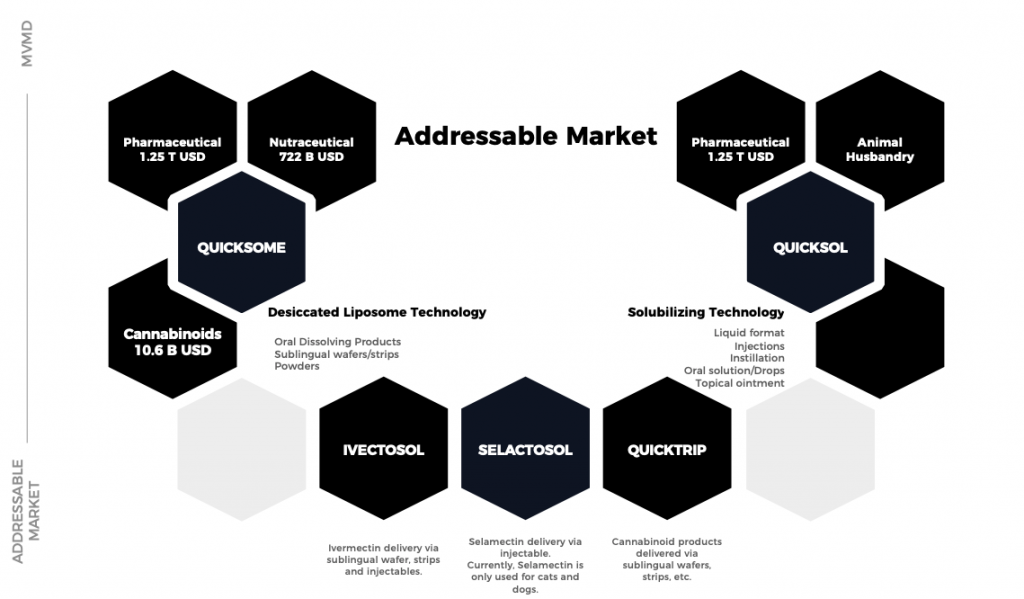
- QuicksomTM – Formulates normally low-bioavailable active ingredients into highly effective oral product formats, improving delivery into the body.
- QuicksolTM – An advanced solubilization technique to create injectable and liquid formulations.
- Dose Sparing Adjuvant – Novel adjuvant (patent pending) with a dose sparing advantage of 10 to 20x.
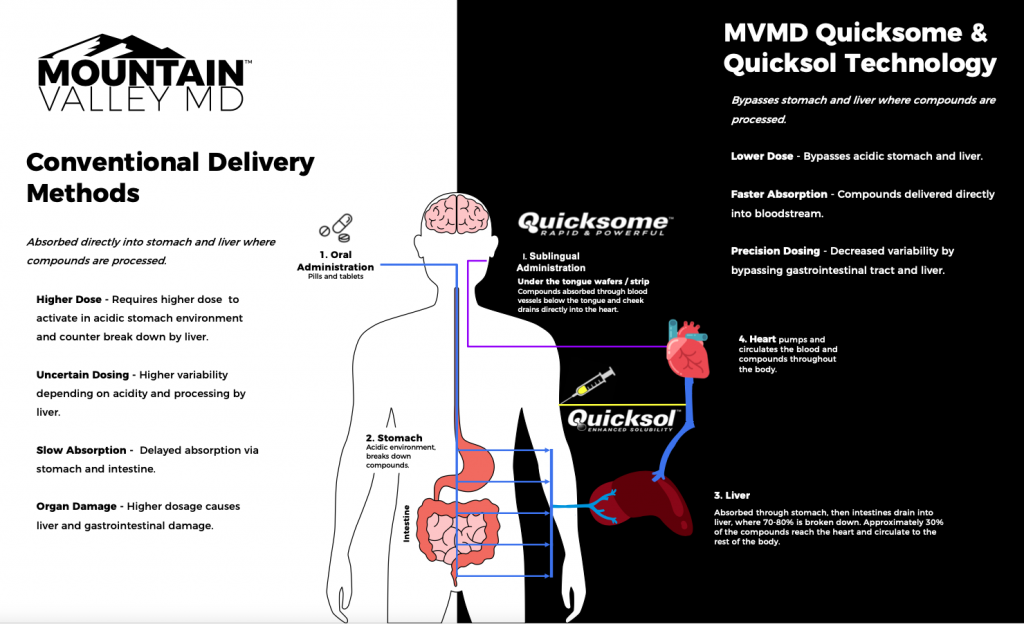

Patented Quicksome™ liposome technology utilizes an advanced 2-step encapsulation and desiccation process to formulate normally highly un-bioavailable active ingredients into highly effective rapid oral dissolving product formats.
It delivers several benefits to the end user, including:
- Fast Acting – Delivers active ingredients into the body faster
- Minimizes Side Effects – Reduces dosing and bypasses liver and gastrointestinal tract
- Rapid Dissolving – Quick water-free oral dissolution
- Needleless – Painless, needle-free administration
- No Pills – Eliminates swallowing and related digestion issues
- Convenient – Easy to store, carry and consume
“Embedding Mountain Valley MD’s Quicksome™️ technology into our unique mushroom product formulations has allowed us to create a product line that we believe will be unmatched in its efficacy in the marketplace… The initial consumer testing with our energy and sleep products has been very well received. The precision of this delivery technology allows us to build the functional mushroom business out while simultaneously pursuing a leadership position in the rapidly emerging psychedelic space.”
– Dr. Sanjeev Goel, Chief Medical Officer at Circadian Wellness and Founder of Peak Human Labs.

Patented Quicksome™ liposome technology utilizes an advanced 2-step encapsulation and desiccation process to formulate normally highly un-bioavailable active ingredients into highly effective rapid oral dissolving product formats.
It delivers several benefits to the end user, including:
- Fast Acting – Delivers active ingredients into the body faster
- Minimizes Side Effects – Reduces dosing and bypasses liver and gastrointestinal tract
- Oral and Injectable – Simplifies administration
- Bioavailability – Increased bioavailability
- Precise Dosing – Eliminates swallowing and related digestion issues
- Eliminate Variability – More accurate absorption into the body, eliminating variability
“We designed the study with the goal of providing the first in vivo proof of enhanced COVID-19 viral clearance ability using both the oral sublingual product and the intramuscular injection product using MVMD’s Quicksol™ technology.”
– Dr. John Clements, Emeritus Professor of Microbiology and Immunology at Tulane University School of Medicine
Dose Sparing Adjuvant
MVMD has developed a novel porous aluminum nanostructures for use as adjuvants in vaccines against various infectious diseases, including polio. These porous aluminum nanostructures have a high surface area for vaccine-antigen binding, provide long-term stability in aqueous media, and promote greater stability in harsh environments.
Adjuvant work (proof-of-concept study in progress at Tulane University) is critical to achieving MVMD’s objective to completely eradicate polio, and additionally this program will inform significant dose sparing applications across hundreds of vaccines, including a sublingual polio vaccine candidate.

Making Existing Drugs Better
IVERMECTIN (Ivectosol): MVMD is currently advancing science with repurposed Ivermectin for other indications including COVID- 19, Malaria, Cancer, Head Lice, Dengue, Zika and Yellow Fever, and a host of husbandry parasitic control applications.
Ivermectin’s ability to modulate immune response paves its path as an adjuvant for cancer therapeutics.
Cancer? Yes, Cancer[10]
COVID-19? Yes, COVID-19[11]
MVMD is commencing preclinical trials to prove Ivectosol’s synergy with certain chemotherapeutics and immunotherapeutics in:
- – Triple negative breast cancer (TNBC)
- – Metastatic melanoma
- – Non-small cell lung carcinoma
- – Non-muscle invasive bladder cancer
Solubilized Ivermectin from MVMD can now be administered intratumorally, intravenously, via infusions and/or instillation.
This has demonstrate significant improvement over the current oral form ivermectin drug.
MVMD’s offering is a waterless oral dissolve, with dramatically quicker onset, zero decline over extended periods in the body, and less variability.
It requires a fraction of the Ivermectin API, uses strictly excipients that are currently approved by the US Food and Drug Administration (FDA), and is leading candidate for human injection and sublingual applications, broader husbandry and companion animal applications based on low viscosity.
MVMD has targeted implications for COVID-19 viral clearance, prophylactic, and broader applications for the elimination of malaria and dengue.
SELAMECTIN (Selactosol): MVMD has successfully solubilized Selamectin as a possible treatment for Tuberculosis.[12]
In 2020, 10 million fell ill from TB and 1.4 million died – a rate of death of 3,836 people per day. The World Health Organization estimates that 1.8 billion people—close to one quarter of the world’s population—are infected with Mycobacterium tuberculosis (M.tb), the bacteria that causes TB.[13]
The global economic cost of TB is estimated to reach over 16 trillion dollarsand kill 75 million people by the year 2050.[14]
Preclinical trials are underway to validate the indication hypothesis. In March of 2021, MVMD achieved solubilization of Selemectin, and filed a new trademark.[15]
Apply their Quicksol™ solubilization technology, MVMD scientists were successful in solubilizing selamectin at 15mg/ml into a water solution without any organic solvents, a critical achievement to allow formulation into topical application creams, oral rapid dissolve sublingual tablets, and injectable applications.
This could be an absolute game changer for the planet, as according to the TB Alliance, Tuberculosis it is the leading infectious cause of death worldwide[16].


Premium Management Team
Mountain Valley MD (CSE:MVMD) (OTC:MVMDF) is led by a well-built team of premium experts in product development, commercialization, and marketing. Among the experts that fill the roster, some examples of the MVMD team’s pedigree includes:
Dennis Hancock – President, CEO, and Director
Mr. Hancock is a senior sales and marketing executive with +25 years of experience spanning automotive, pharmaceutical, tech, telco, retail and financial services sectors. He’s spent +12 years in a leadership role at one of North America’s leading performance improvement and Loyalty providers, Maritz, who works with 70% of the world’s Super 50 companies. Previously, Dennis led publicly traded ZENN Motor Company as the Vice President of Sales and Marketing. As a senior officer at ZMC, Dennis drove the establishment of ZENN – (Zero Emission, No Noise) as one of the most recognized “green tech” brands in North America.
Mike Farber – Director of Delivery Sciences
Mr. Farber is MVMD’s lead inventor and principal scientist. Utilizing his background in biochemistry, he began his career in research and development doing polymer research for container-packaging company Consolidated Bathurst in Montreal. Farber has focused his attention primarily in the area of novel delivery systems for both nutraceuticals and pharmaceuticals. Over the last two decades he’s authored 100+ patents, mainly focused on improving the bioavailability of compounds to enhance effectiveness, efficiency and convenience. His most important current developments include the patented desiccated liposomal rapid dissolve delivery system, macrocyclic lactone drug solubilization and novel dose sparing adjuvant.
Dr. Azhar Rana, MD – Chief Medical Officer
Dr. Rana is the President of Integrated Medhealth Communication (IMC) North America and is a trained general medicine practitioner with a clinical background. He brings to the Company nearly two decades of life sciences experience in clinical development, pre and post launch medical affairs, regulatory and commercial strategy, including 10 years of pharmaceutical leadership at Bristol Myers-Squibb, Novo Nordisk and AstraZeneca. During his pharmaceutical career, he’s gained experience in the development, launch, and life cycle management of novel therapeutics, leading and collaborating with teams in clinical operations and development, medical affairs, regulatory affairs, quality, and pharmacovigilance.
Nancy Richardson – Director
Ms. Richardson is a veteran of the pharmaceutical and agency world, developed continuing medical education for physicians, pharmacists and nurses for over two decades. For 12 years, she has co-run a successful multi-million-dollar medical communications agency, bringing numerous drugs to market, overseeing accounts, generating sales and managing daily operations. She currently serves on the Board of Directors of the Institute of Cultural Affairs Canada.
Paul Lockhard – Director
Mr.Lockhard is a business leader and entrepreneur with +35 years’ experience in consumer goods and digital marketing for such brands as Trident Gum, Energizer Batteries, Ford, Lenscrafters, Labatt Breweries and Guardian Capital. Over the past 19 years, he’s founded four successful businesses, and helped 200+ startups. He’s currently Chief Client Officer at William Thomas Digital, a CRM agency in Toronto serving major Canadian and global companies in retail, loyalty and consumer goods.
Sid Senroy – Scientific Advisor
Mr. Senroy is a seasoned pharmaceutical executive with expertise in helping companies pass compliance assessments, develop robust quality systems and prepare for U.S. Food and Drug Administration reviews and inspections. Over the past two decades, he’s successfully led several global Quality and Compliance business units as an executive or senior consultant, leading to the approval of key blockbuster drugs with cumulative annual sales exceeding $30 billion.
Dr. John Clements, PHD – Scientific Advisor
Dr. Clements is Emeritus Professor of Microbiology and Immunology at Tulane University School of Medicine. He brings invaluable expertise and advisory capacity with +35 years’ experience in vaccine, immunology and infectious diseases research and development. He’s currently helping to advance MVMD’s ongoing QuicksomeTM sublingual polio vaccine development activities. Dr. Clements’ distinguished scientific career has focused on developing and evaluating vaccines for a wide range of infectious diseases globally (including diarrheal diseases, Polio and HIV), including involvement in academia, research and development, governmental and vaccine advisory boards and professional journals. He’s published more than 150 peer-reviewed papers, has 14 issued patents, and has been involved in numerous vaccine clinical trials. He’s worked with leading vaccine focused organizations including the Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), National Institutes of Health (NIH), and the United States Military.
Dr. Michel Rondeau – Scientific Advisor
Dr. Rondeau, oversees MVMD’s husbandry and companion animal studies, while driving global pharmaceutical animal applications as part of the ongoing business commercialization of MVMD’s technology. He’s extensive experience in veterinary research having worked with numerous pharmaceutical companies in animal drug field trials and is credited with co-inventing a global award winning sprayable vaccination device that was acquired by Rhone Poulenc. He’s completed an extensive range of research and development projects across a diverse range of husbandry animals including porcine industrial medicine across preventative and curative medicine, nutrition and animal health products and automated feed systems.

DISCLAIMER:
Nothing in this publication should be considered as personalized financial advice. We are not licensed under securities laws to address your particular financial situation. No communication by our employees to you should be deemed as personalized financial advice. Please consult a licensed financial advisor before making any investment decision. This is a paid advertisement and is neither an offer nor recommendation to buy or sell any security. We hold no investment licenses and are thus neither licensed nor qualified to provide investment advice. The content in this report or email is not provided to any individual with a view toward their individual circumstances. USA News Group is a wholly-owned subsidiary of Market IQ Media Group, Inc. (“MIQ”). MIQ has been paid a fee for Mountain Valley MD. advertising and digital media from USA News Group (“the Company”). There may be 3rd parties who may have shares of Mountain Valley MD, and may liquidate their shares which could have a negative effect on the price of the stock. This compensation constitutes a conflict of interest as to our ability to remain objective in our communication regarding the profiled company. Because of this conflict, individuals are strongly encouraged to not use this publication as the basis for any investment decision. The owner/operator of MIQ own shares of Mountain Valley MD, which were purchased as a part of a private placement. MIQ will not buy or sell shares of Mountain Valley MD for a minimum of 72 hours from the publication date on this website (ENTER DATE), but reserve the right to buy and sell, and will buy and sell shares of Mountain Valley MD at any time thereafter without any further notice. We also expect further compensation as an ongoing digital media effort to increase visibility for the company, no further notice will be given, but let this disclaimer serve as notice that all material disseminated by MIQ has been approved by the above mentioned company; this is a paid advertisement, and we own shares of the mentioned company that we will sell, and we also reserve the right to buy shares of the company in the open market, or through further private placements and/or investment vehicles.
While all information is believed to be reliable, it is not guaranteed by us to be accurate. Individuals should assume that all information contained in our newsletter is not trustworthy unless verified by their own independent research. Also, because events and circumstances frequently do not occur as expected, there will likely be differences between any predictions and actual results. Always consult a licensed investment professional before making any investment decision. Be extremely careful, investing in securities carries a high degree of risk; you may likely lose some or all of the investment.
SOURCES CITED:
[1] https://finance.yahoo.com/news/158-billion-drug-delivery-systems-140800827.html
[2] https://www.q1scientific.com/the-importance-of-vaccine-cold-chain-logistics/
[4] https://www.nature.com/articles/s41392-020-00213-8
[5] https://academic.oup.com/bmb/article/137/1/13/6124816
[6] https://www.forbes.com/sites/beximco-group/2021/09/01/setting-the-bar-high-for-generic-medicines/?sh=6d4b5ecb124d
[7] https://www.cnn.com/2021/02/27/health/covid-19-drug-repurposing-gupta/index.html
[8] https://fortune.com/2018/03/27/viagra-anniversary-pfizer/
[9] https://www.theguardian.com/science/2019/jun/09/race-to-replace-viagra-patents-erectile-dysfunction-drug-medical-research-cialis-eroxon
[10] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7505114/
[11] https://c19ivermectin.com/
[12] https://www.ijidonline.com/article/S1201-9712(14)00900-X/fulltext
[13] https://www.international.gc.ca/world-monde/issues_development-enjeux_developpement/global_health-sante_mondiale/tuberculosis-tuberculose.aspx?lang=eng
[14] https://www.reuters.com/article/us-health-tuberculosis-economy-idUSKBN0MK00520150324
[15] https://www.mountainvalleymd.com/updates/mvmd-achieves-solubilization-of-selemectin-drug-files-new-trademark
[16] https://www.tballiance.org/why-new-tb-drugs/global-pandemic#:~:text=It%20is%20the%20leading%20infectious,TB%20and%201.4%20million%20died.





























