A research team led by Dr. Minah Lee of the Energy Storage Research Center at the Korea Advanced Institute of Science and Technology (KIST) has developed a chemical activation strategy of magnesium metal that enables efficient operation of magnesium batteries in common electrolytes that are free of corrosive additives, and can be mass-produced. The research is published in the journal ACS Nano.
While the demand for lithium-ion batteries is exploding due to the rapid growth of the electric vehicle and energy storage system (ESS) markets, the supply and demand of their raw materials such as lithium and cobalt are mostly dependent on specific countries, and thus there are great concerns about securing a stable supply chain. For this reason, research on next-generation secondary batteries have been actively conducted, and secondary batteries utilizing magnesium, which is abundant in the earth’s crust, are gaining attention.
Magnesium secondary batteries can be expected to have a high energy density because they utilize Mg2+, a divalent ion instead of monovalent alkali metal ions such as lithium. The highest energy density can be obtained by directly utilizing magnesium metal as a anode, of which volumetric capacity is about 1.9 times higher than lithium metal.
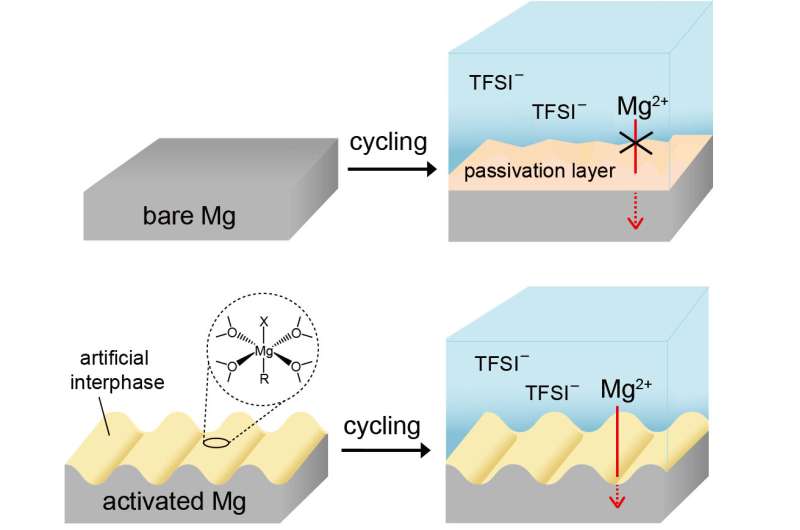
Despite these advantages, the difficulty of efficiently charging and discharging magnesium metal due to its reactivity with electrolytes, has hindered its commercialization. KIST researchers have developed a technology to induce a highly efficient charge and discharge reaction of magnesium metal, opening the possibility of the commercialization of magnesium secondary batteries.
In particular, unlike previous studies that utilized corrosive electrolytes to facilitate the charging and discharging of magnesium, the researchers utilized a common electrolyte with a similar composition to existing commercial electrolytes, enabling the use of high-voltage electrodes and minimizing corrosion of battery components.
The team synthesized an artificial protective layer with a novel composition based on magnesium alkyl halide oligomers on the magnesium surface by a simple process of dipping the magnesium metal to be used as the anode into a reactive alkyl halide solution prior to cell assembly. They found that selecting a specific reaction solvent facilitated the formation of nanostructures on the magnesium surface, which in turn facilitated the dissolution and deposition of magnesium.
Based on this, they suppressed unwanted reactions with electrolytes and maximized the reaction area through nanostructuring to induce highly efficient magnesium cycling.
By applying the developed technology, the overpotential can be reduced from more than 2 V to less than 0.2 V when charging and discharging magnesium metal in a common electrolyte without corrosive additives, and the Coulombic efficiency can be increased from less than 10% to more than 99.5%. The team demonstrated stable charging and discharging of activated magnesium metal more than 990 cycles, confirming that magnesium rechargeable batteries can operate in conventional electrolytes that can be mass-produced.
“This work provides a new direction for the existing magnesium secondary battery research, which has been using corrosive electrolytes that prevent the formation of interfacial layers on magnesium metal surfaces,” said Dr. Minah Lee of KIST. “It will increase the possibility of low-cost, high-energy-density magnesium secondary batteries based on common electrolytes suitable for energy storage systems (ESS).”