Li-ion batteries, rechargeable batteries based on lithium ions, are currently used to power a wide range of electronic devices, ranging from smartphones to portable computers, toys, wireless headphones, and electric vehicles. Despite their remarkable performances, these batteries are made using some unsustainable and expensive raw materials.
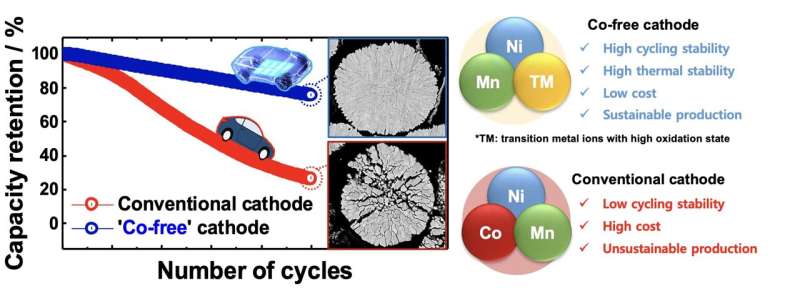
The most notable among these materials is cobalt (Co), which is used to create layered cathodes for Li-ion batteries. Because of the recent surge in demand for electric vehicles, cobalt is rapidly becoming scarce on Earth, also due to the rising demand from the technology and electronics industry.
Researchers at Hanyang University and the Pacific Northwest National Laboratory have recently shown that creating highly performing layered cathodes without using Co could in fact be possible. Their paper, published in Nature Energy, could ultimately contribute to the development of more sustainable and affordable Li-ion battery solutions.
“Prof. Sun and I have been working together on cathode materials for the past 20 years,” Prof. Chong S. Yoon, one of the researchers who carried out the study, told TechXplore. “With the increasing depletion of Co and its supply uncertainty, we recognized that it is becoming increasingly imperative to eliminate Co from Ni-rich layered cathodes (NCA or NCM) used in electric vehicles.”
So far, eliminating Co from layered cathodes for Li-ion batteries has proved extremely challenging. This is because even a small amount of this material can significantly improve the structural stability of cathodes, which in turn expedites the so-called Li-intercalation kinetics. This is an essential chemical process that ultimately enables high performances in batteries. To overcome this limitation, some researchers have been exploring the potential of a cathode made of Li(NixMn1-x)O2; arguably the most straightforward cathode-free composition.
“Li(Ni0.5Mn0.5)O2 is a well-studied Co-free cathode with stable cycling stability, but it provides insufficient capacity for today’s EVs,” Yoon said. “There have been attempts to increase the Ni content in Li(Ni0.5Mn0.5)O2 to increase its capacity, but the capacity problem remained unresolved. Without Co, it is difficult to extract Li from the host structure.”
To overcome the challenges encountered during previous attempts at creating highly performing Li(Ni0.5Mn0.5)O2 cathodes, Yoon and his colleagues increased their cathodes’ operating voltage from 4.3 V to 4.4 V. This allowed them to extract a larger fraction of Li from Li(Ni0.9Mn0.1)O2 , which simultaneously increased the energy density and power density at the batteries’ highest operating voltage. To ensure that their Li-ion battery remained stable at 4.4V (the highest operating voltage), the team then had to re-engineer the batteries’ cathode microstructure and electrolyte.
“From our experience, we knew that introducing dopants with high oxidation states (Mo, W, Sb, Ta, etc.) refines the primary particle size and stabilizes the delithiated host structure,” Yoon said. “We took a heuristic approach based on our past experience to determine that doping the Li(Ni0.9Mn0.1)O2 cathode with 1 mol% Mo gives the best performance. Fluoroethylene carbonate was also added to the conventional electrolyte to support the electrolyte at 4.4 V and protect the cathode surface from electrolyte attack. 1 mol% Mo—Li(Ni0.9Mn0.1)O2 cycled 1,000 times while retaining 86% of the initial capacity which is more than sufficient to meet the battery life specified by the electric vehicle manufacturer.”
The main difficulty that Yoon and his colleagues encountered when trying to operate their 1 mol% Mo—Li(Ni0.9Mn0.1)O2 cathode at 4.4 V was capacity fading during prolonged cycling (i.e., a loss of capacity over time that plagues all rechargeable batteries based on Ni-rich layered cathodes). To ensure that their battery had enough life to power devices for a reasonable amount of time, they first had to resolve this capacity fading issue.
“The exceptional cycling stability of 1 mol% Mo—Li(Ni0.9Mn0.1)O2 at 4.4 V is largely owed to the grain size refinement and cation ordering,” Yoon explained. “Mo6+ ions tend to segregate along interparticle boundaries and inhibit grain growth during high-temperature heat treatment which is necessary to convert the hydroxide precursor to Li(Ni0.9Mn0.1)O2. Grain boundaries in this ultrafine-structured cathode increase fracture toughness by deflecting cracks generated by the abrupt lattice contraction near the charge end.”
The grain boundaries in the researchers’ cathode can also act as fast diffusion paths for Li+, which remove local inhomogeneities due to their composition and suppress intragranular fractures. By introducing Mo6+, the team was able to arrange cations in their cathode in a specific way (i.e., intermixing Li and Ni ions). This unique design stabilizes the cathode’s structure when it is most vulnerable due to the non-uniform extraction of Li+ ions.
“Our findings suggest that developing a high-performance Co-free layered cathode is no-longer an elusive goal,” Yoon said. “The proposed 1 mol% Mo—Li(Ni0.9Mn0.1)O2cathode cycling at a high voltage is an economically viable solution that is achievable with current manufacturing technology. In addition, by clarifying the intrinsic role of Co during the extraction of Li+ from the host structure, this work offers a material design criterion for selecting a tertiary doping element for ensuring the structural and mechanical durability of Co-free layered cathodes.”
The cathode design and composition proposed by Yoon and his colleagues could guide future research efforts aimed at improving the general performance of Ni-rich layered cathodes. In addition, their work might pave the way towards the creation of highly performing Co-free battery technologies, which could be more sustainable and affordable that their Co-based counterparts.
“For high-performance EVs with a long driving range and improved safety, the next generation Li-ion batteries will likely be all-solid-state batteries (ASSB) featuring Ni-rich Co-free cathodes,” Yoon added. “We are now investigating the possibility of applying the proposed Co-free cathode to ASSB.”